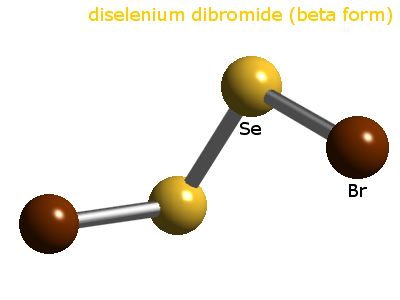
Selenium dibromide is a compound made of one selenium and two bromine atoms. It is unstable. No solid form of the compound has been discovered but it is a component of the equilibria in the vapour above selenium tetrabromide (SeBr4) and in nonaqueous solutions. In acetonitrile solution, selenium reacts with SeBr4 to form an equilibrium mixture containing SeBr2, Se2Br2 and Br2. This covalent compound has a bent molecular geometry in the gas phase.
References