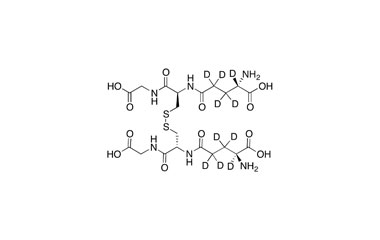
Glutathione disulfide (GSSG) is a disulfide derived from two glutathione molecules.
In living cells, glutathione disulfide is reduced into two molecules of glutathione with reducing equivalents from the coenzyme NADPH. This reaction is catalyzed by the enzyme glutathione reductase.
Antioxidant enzymes, such as glutathione peroxidases and peroxiredoxins, generate glutathione disulfide during the reduction of peroxides such as hydrogen peroxide (H2O2) and organic hydroperoxides (ROOH):
- 2 GSH ROOH → GSSG ROH H2O
Other enzymes, such as glutaredoxins, generate glutathione disulfide through thiol-disulfide exchange with protein disulfide bonds or other low molecular mass compounds, such as coenzyme A disulfide or dehydroascorbic acid.
- 2 GSH R-S-S-R → GSSG 2 RSH
The GSH:GSSG ratio is therefore an important bioindicator of cellular health, with a higher ratio signifying less oxidative stress in the organism. A lower ratio may even be indicative of neurodegenerative diseases, such as Parkinson's disease (PD) and Alzheimer's disease.
Neuromodulator
GSSG, along with glutathione and S-nitrosoglutathione (GSNO), have been found to bind to the glutamate recognition site of the NMDA and AMPA receptors (via their γ-glutamyl moieties), and may be endogenous neuromodulators. At millimolar concentrations, they may also modulate the redox state of the NMDA receptor complex.
See also
- Glutathione-ascorbate cycle
- Antioxidant
References